-
Structural, Photophysical and Magnetic Properties of Transition Metal Complexes Based on the Dipicolylamino-chloro-1,2,4,5-tetrazine Ligand

I. Nazarenko, F. Pop, Q. Sun, A. Hauser, F. Lloret, M. Julve, A. El-Ghayoury and N. Avarvari
Dalton Transactions, 44 (19) (2015), p8855-8866


DOI:10.1039/c5dt00550g | unige:72655 | Abstract | Article HTML | Article PDF | Supporting Info
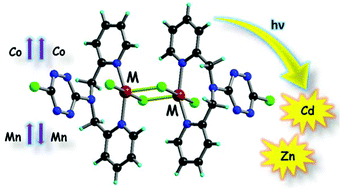
The ligand 3-chloro-6-dipicolylamino-1,2,4,5-tetrazine (Cl-TTZ-dipica) 1, prepared by the direct reaction between 3,6-dichloro-1,2,4,5-tetrazine and di(2-picolyl)-amine, afforded a series of four neutral transition metal complexes formulated as [Cl-TTZ-dipica-MCl2]2, with M = Zn(II)Â 2a, Cd(II) 2b, Mn(II) 2c and Co(II) 2d, when reacted with the corresponding metal chlorides. The dinuclear structure of the isostructural complexes was disclosed by single crystal X-ray analysis, clearly indicating the formation of [MII-(m-Cl)2MII] motifs and the involvement of the amino nitrogen atom in semi-coordination with the metal centers, thus leading to distorted octahedral coordination geometries. Moreover, the chlorine atoms, either coordinated to the metal or as substituent on the tetrazine ring, engage respectively in specific anion-p intramolecular and intermolecular interactions with the electron poor tetrazine units in the solid state, thus controlling the supramolecular architecture. Modulation of the emission properties is observed in the case of the Zn(II) and Cd(II) complexes when compared to the free ligand. A striking difference is observed in the magnetic properties of the Mn(II) and Co(II) complexes. An antiferromagnetic coupling takes place in the dimanganese(II) compound (J = -1.25 cm-1) while the Co(II) centers are ferromagnetically coupled in the corresponding complex (J = +0.55 cm-1), the spin Hamiltonian being defined as H = -JSA.SB.